Diacetyliminoxyl as a selective radical reagent for organic synthesis: dehydrogenation and dehydrogenative C–O coupling reactions†
Abstract
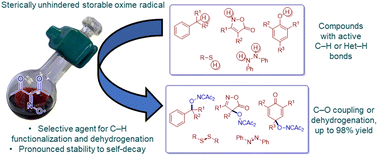
The field of free-radical reagents in organic chemistry has been associated with aminoxyl radicals (mainly TEMPO and its analogues) for a long time. Diacetyliminoxyl is the first readily synthetically available oxime radical, which differs sharply from aminoxyl radicals in structure and reactivity and exhibits an outstanding stability to self-decay compared to other sterically unhindered oxime radicals. Herein we introduce diacetyliminoxyl as a novel radical reagent for organic synthesis and demonstrate its application in oxidative functionalization with the cleavage of OH, CH, NH and SH bonds and dehydrogenation processes. It was found that diacetyliminoxyl is a highly selective hydrogen abstracting reagent towards activated substrates or functional groups. It was also shown that diacetyliminoxyl is an exceptionally effective interceptor of stabilized and sterically hindered C-radicals which are not trapped by a typical radical scavenger like TEMPO.


 Please wait while we load your content...
Please wait while we load your content...