Restricted rotation and tunable fluorescence in atropisomeric naphthyl pyridine chromophores†
Abstract
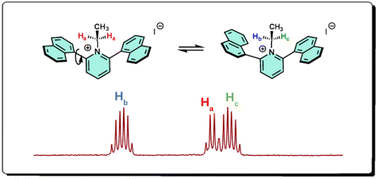
Enhanced fluorescence quantum yields are enabled by simple reactions at the heterocyclic nitrogen in naphthyl-pyridine chromophores in which the electronic properties can be tuned through protonation, oxidation, and alkylation at the nitrogen center. Fluorescence quantum yield is increased by reacting the pyridine lone pair with either a proton or an alkyl group. Restricted intramolecular rotation (RIR) was observed upon alkylation, as evidenced by the presence of atropisomers. These simple structural changes allow application-driven tuning of electronic properties.


 Please wait while we load your content...
Please wait while we load your content...