π-Facial selectivity in the Diels–Alder reaction of glucosamine-based chiral furans and maleimides†
Abstract
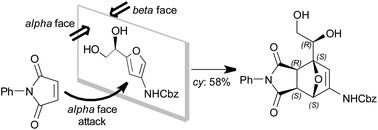
Furans derived from carbohydrate feedstocks are a versatile class of bio-renewable building blocks and have been used extensively to access 7-oxanorbornenes via Diels–Alder reactions. Due to their substitution patterns these furans typically have two different π-faces and therefore furnish racemates in [4 + 2]-cycloadditions. We report the use of an enantiopure glucosamine derived furan that under kinetic conditions predominantly affords the exo-product with a high π-face selectivity of 6.5 : 1. The structure of the product has been resolved unequivocally by X-ray crystallography, and a multi-gram synthesis (2.8 g, 58% yield) confirms the facile accessibility of this multifunctional enantiopure building block.


 Please wait while we load your content...
Please wait while we load your content...