Development of phenazine-2,3-diol-based photosensitizers: effect of substitution of the cyano group for the nitro group on singlet oxygen generation†
Abstract
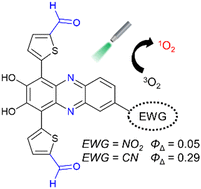
Cyano-substituted phenazine-2,3-diol-based dyes, YC-1 and YC-2, which have n-hexyl and formyl groups, respectively, on thiophene units, have been newly developed as halogen-atom-free-heteroanthracene-based photosensitizers (PSs) possessing the ability to generate singlet oxygen (1O2). The photoabsorption edge (λabsedge) of YC-1 reached 700 nm which is in a longer wavelength region by 50 nm in comparison with that (650 nm) of YC-2. However, the λabsedge of YC-1 and YC-2 exhibited blue-shifts, compared to those (700 nm and 800 nm) of nitro-substituted phenazine-2,3-diol-based dyes KI-3 and KI-5 with formyl groups and the n-hexyl group, respectively, in our previous work. KI-3 and KI-5 show low fluorescence and 1O2 generation quantum yields (Φfl < 0.01 and ΦΔ = 0.05 and 0.02, respectively), which are due to the rapid nonradiative decay of the excited states by the nitro group. YC-1 and YC-2 exhibited also feeble fluorescence with low Φfl values (<0.01 and 0.022, respectively), meanwhile YC-2 showed a moderate ΦΔ value (0.29) but the ΦΔ value (0.0081) of YC-1 was significantly lower than those of the other PSs. Time-dependent density functional theory (TDDFT) calculations revealed that YC-2 with the formyl groups has the smallest energy gap ΔEST (−0.05 eV) between the S1 (ππ*) state and the Tn state with nπ* characteristics among these PSs (−0.12 eV for KI-3, −0.36 eV for KI-5, and −0.24 eV for YC-1), leading to efficient intersystem crossing (ISC) according to the El-Sayed rule. In addition, it was suggested that the low ΦΔ value of YC-1 with n-hexyl groups is due to the relatively large ΔEST value as well as the flexible n-hexyl groups which accelerate internal conversion (IC) from the S1 to S0 state, as evidenced by the low Φfl values, resulting in inferior ISC. Consequently, we demonstrated that cyano-substituted phenazine-2,3-diol-based PSs reduce the rapid nonradiative decay of the excited states and the ΔEST values, leading to efficient ISC for 1O2 generation, compared to nitro-substituted phenazine-2,3-diol-based PSs.


 Please wait while we load your content...
Please wait while we load your content...