Direct measurements of size-independent lithium diffusion and reaction times in individual polycrystalline battery particles†
Abstract
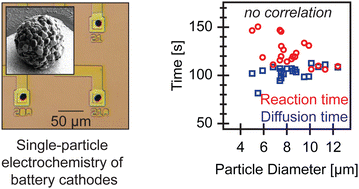
Polycrystalline Li(Ni,Mn,Co)O2 (NMC) secondary particles are the most common cathode materials for Li-ion batteries. During electrochemical (dis)charge, lithium is believed to diffuse through the bulk and enter (leave) the secondary particle at the surface. Based on this model, smaller particles would cycle faster due to shorter diffusion lengths and larger surface-area-to-volume ratios. In this work, we evaluate this widespread assumption by developing a new high-throughput single-particle electrochemistry platform using the multi-electrode array from neuroscience. By measuring the reaction and diffusion times for 21 individual particles in liquid electrolytes, we find no correlation between the particle size and either the reaction or diffusion times, which is in stark contrast to the prevailing lithium transport model. We propose that electrochemical reactions occur inside secondary particles, likely due to electrolyte penetration into cracks. Our high-throughput, single-particle electrochemical platform further opens new frontiers for robust, statistical quantification of individual particles in electrochemical systems.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...