On a high-capacity aluminium battery with a two-electron phenothiazine redox polymer as a positive electrode†
Abstract
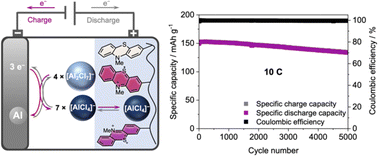
With aluminium being the most abundant metal in Earth's crust, rechargeable Al ion batteries (AIBs) hold great promise as next-generation energy storage devices. However, the currently used positive electrode materials suffer from low specific capacity, which limits the specific energies of these AIBs. Here, we present an organic redox polymer with two well-defined redox processes as a positive electrode material that overcomes these shortcomings. Cross-linked poly(3-vinyl-N-methylphenothiazine) with phenothiazine as a two-electron redox centre reversibly inserts [AlCl4]− ions at potentials of 0.81 and 1.65 V vs. Al|Al3+, delivers experimental specific capacities of up to 167 mA h g−1 in AIBs and surpasses graphite as a positive electrode material. After 5000 cycles at a 10C rate, this AIB retains 88% of its capacity. Even at a 100C rate, 64 mA h g−1 can be reversibly cycled, and the AIB returns to its original capacity without any changes at slower rates. This is the first report of a reversible two-electron redox process for a phenothiazine-based battery electrode material. With its high discharge voltage and specific capacity, and its excellent capacity retention at fast C-rates combined with flat charge/discharge plateaus, this AIB plays a major role in the development of rechargeable AIBs and will initiate further explorations of organic redox polymers as positive electrode materials, paving the way towards more sustainable energy storage devices.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...