Impact of the central atom and halido ligand on the structure, antiproliferative activity and selectivity of half-sandwich Ru(ii) and Ir(iii) complexes with a 1,3,4-thiadiazole-based ligand†
Abstract
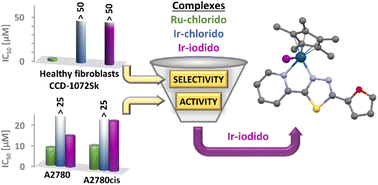
Half-sandwich complexes [Ru(η6-pcym)(L1)X]PF6 (1, 3) and [Ir(η5-Cp*)(L1)X]PF6 (2, 4) featuring a thiadiazole-based ligand L1 (2-(furan-2-yl)-5-(pyridin-2-yl)-1,3,4-thiadiazole) were synthesized and characterized by varied analytical methods, including single-crystal X-ray diffraction (X = Cl or I, pcym = p-cymene, Cp* = pentamethylcyclopentadienyl). The structures of the molecules were analysed and interpreted using computational methods such as Density Functional Theory (DFT) and Quantum Theory of Atoms in Molecules (QT-AIM). A 1H NMR spectroscopy study showed that complexes 1–3 exhibited hydrolytic stability while 4 underwent partial iodido/chlorido ligand exchange in phosphate-buffered saline. Moreover, 1–4 demonstrated the ability to oxidize NADH (reduced nicotinamide adenine dinucleotide) to NAD+ with Ir(III) complexes 2 and 4 displaying higher catalytic activity compared to their Ru(II) analogues. None of the complexes interacted with reduced glutathione (GSH). Additionally, 1–4 exhibited greater lipophilicity than cisplatin. In vitro biological analyses were performed in healthy cell lines (CCD-18Co colon and CCD-1072Sk foreskin fibroblasts) as well as in cisplatin-sensitive (A2780) and -resistant (A2780cis) ovarian cancer cell lines. The results indicated that Ir(III) complexes 2 and 4 had no effect on human fibroblasts, demonstrating their selectivity. In contrast, complexes 1 and 4 exhibited moderate inhibitory effects on the metabolic and proliferation activities of the cancer cells tested (selectivity index SI > 3.4 for 4 and 2.6 for cisplatin; SI = IC50(A2780)/IC50(CCD-18Co)), including the cisplatin-resistant cancer cell line. Based on these findings, it is possible to emphasize that mainly complex 4 could represent a further step in the development of selective and highly effective anticancer agents, particularly against resistant tumour types.


 Please wait while we load your content...
Please wait while we load your content...