Manganese(iii) complexes stabilized with N-heterocyclic carbene ligands for alcohol oxidation catalysis†
Abstract
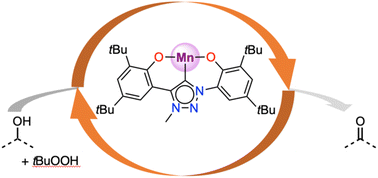
The chemistry of N-heterocyclic carbenes with Earth-abundant manganese has largely focused on low-valent systems for reductive catalysis. Here, we have decorated imidazole- and triazole-derived carbenes with phenol substituents to access higher-valent Mn(III) complexes [Mn(O,C,O)(acac)], where acac = acetylacetonato, and O,C,O = bis(phenolate)imidazolylidene (1) or bis(phenolate)triazolylidene (2). Both complexes catalyze the oxidation of alcohols in the presence of tBuOOH as terminal oxidant. Complex 2 is slightly more active than 1 (TOF up to 540 h−1vs. 500 h−1), yet significantly more robust towards deactivation. Secondary and primary alcohols are oxidized, the latter with high selectivity and essentially no overoxidation of the aldehyde product to carboxylic acids unless the reaction time is substantially extended. Mechanistic investigations using Hammett parameters, IR spectroscopy, isotope labelling experiments, and specific substrates and oxidants as probes support the formation of a manganese(V) oxo system as the active species and subsequent turnover-limiting hydrogen atom abstraction.


 Please wait while we load your content...
Please wait while we load your content...