Electrocatalytic reduction of CO2 to CO by a series of organometallic Re(i)-tpy complexes†
Abstract
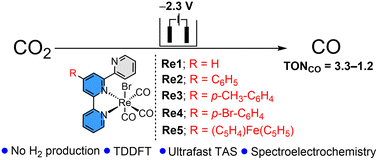
A series of organometallic Re(I)(L)(CO)3Br complexes with 4′-substituted terpyridine ligands (L) has been synthesised as electrocatalysts for CO2 reduction. The complexes’ spectroscopic characterisation and computationally optimised geometry demonstrate a facial geometry around Re(I) with three cis COs and the terpyridine ligand coordinating in a bidentate mode. The effect of substitution on the 4′-position of terpyridine (Re1–5) on CO2 electroreduction was investigated and compared with a known Lehn-type catalyst, Re(I)(bpy)(CO)3Br (Re7). All complexes catalyse CO evolution in homogeneous organic media at moderate overpotentials (0.75–0.95 V) with faradaic yields of 62–98%. The electrochemical catalytic activity was further evaluated in the presence of three Brønsted acids to demonstrate the influence of the pKa of the proton sources. The TDDFT and ultrafast transient absorption spectroscopy (TAS) studies showed combined charge transfer bands of ILCT and MLCT. Amongst the series, the Re-complex containing a ferrocenyl-substituted terpyridine ligand (Re5) shows an additional intra-ligand charge transfer band and was probed using UV-Vis spectroelectrochemistry.
- This article is part of the themed collection: New Talent: Asia Pacific


 Please wait while we load your content...
Please wait while we load your content...