First ternary tungsten tellurate(iv) WTe2O7 with unique crystal structure type†
Abstract
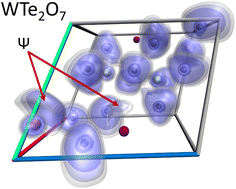
At multianvil high-pressure/high-temperature conditions of 10 GPa and 1273 K, the first ternary tungsten tellurate WTe2O7 is formed, starting from a stoichiometric mixture of WO3 and TeO2. The compound crystallizes triclinic in a hitherto unknown crystal structure type with the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; (no. 2), and was refined from single-crystal X-ray diffractometer data: a = 538.3(1), b = 687.5(1), c = 802.3(1) pm, α = 72.4(1)°, β = 85.7(1)°, γ = 68.1(1)°, wR2 = 0.0323, GooF = 1.048, 3157 F2 values, and 106 variables. The main motifs of the crystal structure are pairs of edge-linked [WO6]6− octahedra and fourfold oxygen-coordinated Te4+ atoms. The oxidation state of W6+ and Te4+ was further verified by measuring the characteristic binding energy values for the W 4f and the Te 3d core levels via X-ray photoelectron spectroscopy (XPS). In addition, DFT calculations of the structure, the associated electron localisation functions (ELF) and vibrational spectra have been carried out. The theoretical data clearly demonstrates the impact of the residual electron density located at the Te4+ ions, which can be directly interpreted as the presence of lone electron pairs within the solid structure.
; (no. 2), and was refined from single-crystal X-ray diffractometer data: a = 538.3(1), b = 687.5(1), c = 802.3(1) pm, α = 72.4(1)°, β = 85.7(1)°, γ = 68.1(1)°, wR2 = 0.0323, GooF = 1.048, 3157 F2 values, and 106 variables. The main motifs of the crystal structure are pairs of edge-linked [WO6]6− octahedra and fourfold oxygen-coordinated Te4+ atoms. The oxidation state of W6+ and Te4+ was further verified by measuring the characteristic binding energy values for the W 4f and the Te 3d core levels via X-ray photoelectron spectroscopy (XPS). In addition, DFT calculations of the structure, the associated electron localisation functions (ELF) and vibrational spectra have been carried out. The theoretical data clearly demonstrates the impact of the residual electron density located at the Te4+ ions, which can be directly interpreted as the presence of lone electron pairs within the solid structure.


 Please wait while we load your content...
Please wait while we load your content...