The role of solvents and concentrations in the properties of oxime bearing A2B corroles†
Abstract
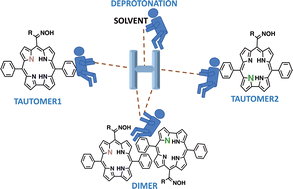
A comprehensive study on the electronic spectral, photophysical and acid–base properties of phenyl- and methyl-oxime corrole derivatives and of triphenylcorrole (model corrole) has been performed, aiming to shed light on the existing species in the ground and excited states. Solvents and corrole concentration are found to govern the properties of the studied compounds and are determinants of their applicability in in vivo studies. In THF, the neutral corrole has two tautomeric forms (T1 and T2). In DMSO, the deprotonated form shows a characteristic long-wavelength Q band slightly shifted to blue when compared with the T1 tautomer and a higher fluorescence quantum yield. In ACN, with the increase of the corrole concentration formation of an aggregate due to homoconjugation (with dimer characteristics) is observed, and pioneeringly reported using UV-Vis and fluorescence studies and confirmed by carrying out titrations with TFA. The effect of the oxime group on the pK values of a corrole is found to influence the formation of a homoconjugate, namely by precluding its formation (at higher concentrations) when compared with the model corrole. TDDFT electronic quantum calculations support the experimental observations, namely the existence of tautomers and deprotonated species, with their respective electronic spectral features, further allowed proposing a structure for the homoconjugate complex in ACN. The characteristics of the oxime-corroles, namely a pK of ∼ 5, absorption and emission at ca. 650 nm and solvent dependent properties, make them good candidates for their use in biological systems either as probes, sensors, or as new sensitizers for photodynamic therapy.


 Please wait while we load your content...
Please wait while we load your content...