Role of electron localisation in H adsorption and hydride formation in the Mg basal plane under aqueous corrosion: a first-principles study†
Abstract
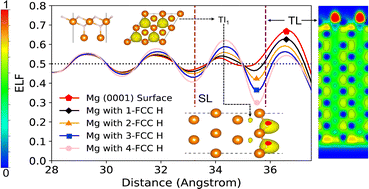
Understanding hydrogen-metal interactions is important in various fields of surface science, including the aqueous corrosion of metals. The interaction between atomic H and a Mg surface is a key process for the formation of sub-surface Mg hydride, which may play an important role in Mg aqueous corrosion. In the present work, we performed first-principles Density Functional Theory (DFT) calculations to study the mechanisms for hydrogen adsorption and crystalline Mg hydride formation under aqueous conditions. The Electron Localisation Function (ELF) is found to be a promising indicator for predicting stable H adsorption in the Mg surface. It is found that H adsorption and hydride layer formation is dominated by high ELF adsorption sites. Our calculations suggest that the on-surface adsorption of atomic H, OH radicals and atomic O could enhance the electron localisation at specific sites in the sub-surface region, thus forming effective H traps locally. This is predicted to result in the formation of a thermodynamically stable sub-surface hydride layer, which is a potential precursor of the crucial hydride corrosion product of magnesium.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...