Why does thionating a carbonyl molecule make it a better electron acceptor?†
Abstract
The past decade has witnessed a surge of biomedical and materials applications of thiocarbonyl molecules (R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), such as in photodynamic therapy, organic field-effect transistors, and rechargeable batteries. The success of these applications originates from thiocarbonyl's small optical gap in the visible region and the enhanced electron affinity compared to the carbonyl analogues (R2C
S), such as in photodynamic therapy, organic field-effect transistors, and rechargeable batteries. The success of these applications originates from thiocarbonyl's small optical gap in the visible region and the enhanced electron affinity compared to the carbonyl analogues (R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Although these observations seem to be contrary to the implication based on a simple electronegativity consideration (2.58 for sulfur and 3.44 for oxygen), a natural bond orbital (NBO) analysis gives a straightforward explanation for the LUMO-lowering effect of C
O). Although these observations seem to be contrary to the implication based on a simple electronegativity consideration (2.58 for sulfur and 3.44 for oxygen), a natural bond orbital (NBO) analysis gives a straightforward explanation for the LUMO-lowering effect of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → C
O → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S substitution. In comparison to the valence (2p)C/(2p)O interactions in C
S substitution. In comparison to the valence (2p)C/(2p)O interactions in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, the higher 3p orbital of sulfur and its weaker overlap with the 2p level of carbon result in a weaker antibonding interaction in
O, the higher 3p orbital of sulfur and its weaker overlap with the 2p level of carbon result in a weaker antibonding interaction in 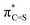 NBO, a prominent contributor to the LUMO. Such an analysis also provides a semi-quantitative understanding of the electronic effect of substituents on or in π-conjugation with a (thio)carbonyl functionality. The intuitive concepts uncovered here offer a simple rule to predict the electronic properties of π-conjugated molecules that incorporate heavy heteroelements and would facilitate materials development.
NBO, a prominent contributor to the LUMO. Such an analysis also provides a semi-quantitative understanding of the electronic effect of substituents on or in π-conjugation with a (thio)carbonyl functionality. The intuitive concepts uncovered here offer a simple rule to predict the electronic properties of π-conjugated molecules that incorporate heavy heteroelements and would facilitate materials development.



 Please wait while we load your content...
Please wait while we load your content...