A DFT study of the endo-selectivity mechanism of the Diels–Alder reaction in lindenane dimeric sesquiterpene synthesis promoted by pyridines†
Abstract
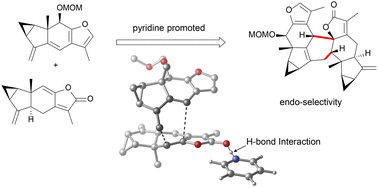
The lindenane dimeric sesquiterpenoids with versatile biological activities are accessible via biometric synthesis, in which the endo-selective Diels–Alder reaction plays an important role. To explore the endo-selectivity of the Diels–Alder reaction between lindenane sesquiterpenes promoted by pyridines, density functional theory (DFT) calculations were performed to explore the reaction mechanism between pyridines and D–A monomers. The calculations performed on the reaction pathways explain why pyridines can promote endo-selectivity via hydrogen bonding, and the hydrogen bond strength is a key factor driving the Diels–Alder reaction in major biochemical systems. These DFT-level insights will pave the way for designing better promoters for Diels–Alder reactions in biometric synthesis applications.


 Please wait while we load your content...
Please wait while we load your content...