Designing scintillating coordination polymers using a dual-ligand synthetic approach†
Abstract
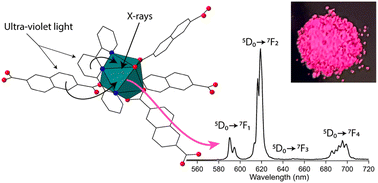
Herein we report the synthesis and structural characterization of three new europium containing coordination polymers: [Eu(terpy)(2,6-ndc)1.5]·H2O (Linus Pauling (LP) 1), [Eu(terpy)(1,4-ndc)(1,4-Hndc)] (LP-2), and [Eu(phen)(2,6-ndc)1.5]·DMF (LP-3). The title compounds were prepared hydro- or solvo-thermally and are constructed from either 1,4- or 2,6-naphthalenedicarboxylic acid (ndc) and 1,10-phenanthroline (phen) or 2,2′:6′,2′′-terpyridine (terpy) ligands. The X-ray diffraction data show LP-2 is two-dimensional while LP-1 and LP-3 are three-dimensional. Compounds LP-1 and LP-2 exhibit modest thermal stability up to 400 °C, while LP-3 was notably unstable when removed from its reaction media and prone to degradation. Despite the apparent porosity, nitrogen physisorption measurements conducted on LP-1 and LP-2 revealed lower than anticipated surface area and pore volumes of 1 m2 g−1 and 0.037 cm3 g−1 and 1 m2 g−1 and 0.015 cm3 g−1, respectively. Photoluminescence characterization of all three compounds was performed in the solid state and in all cases the results reveal bright Eu3+-based emission. The excitation spectra show the emissive properties arise from efficient ligand-to-metal resonance energy transfer. Additionally, LP-1 and LP-2 also display emission when exposed to soft X-rays, and as such show promise toward the design of scintillators.


 Please wait while we load your content...
Please wait while we load your content...