Synthesis of functionalized spirocyclic oxetanes through Paternò–Büchi reactions of cyclic ketones and maleic acid derivatives†
Abstract
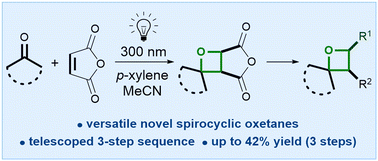
A telescoped three-step sequence to functionalised spirocyclic oxetanes is reported, involving Paternò–Büchi reactions between maleic acid derivatives and cyclic ketones. p-Xylene suppresses the competing alkene dimerization that has plagued previous work, allowing access to 35 novel spirocyclic oxetanes that cannot be prepared using existing methodologies, and which represent versatile intermediates for further elaboration.


 Please wait while we load your content...
Please wait while we load your content...