Biosynthetic incorporation of fluorinated amino acids into the nonribosomal peptide gramicidin S†
Abstract
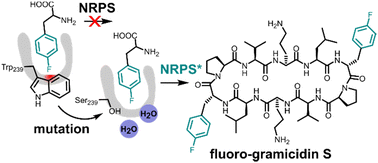
Fluorine is a key element in medicinal chemistry, as it can significantly enhance the pharmacological properties of drugs. In this study, we aimed to biosynthetically produce fluorinated analogues of the antimicrobial cyclic decapeptide gramicidin S (GS). However, our results show that the A-domain of the NRPS module GrsA rejects 4-fluorinated analogues of its native substrate Phe due to an interrupted T-shaped aromatic interaction in the binding pocket. We demonstrate that GrsA mutant W239S improves the incorporation of 4-fluorinated Phe into GS both in vitro and in vivo. Our findings provide new insights into the behavior of NRPSs towards fluorinated amino acids and strategies for the engineered biosynthesis of fluorinated peptides.
- This article is part of the themed collection: 2023 RSC Chemical Biology Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...