Synthesis, self-assembly and optical properties of some rigid π-bridged triphenylene dimers†
Abstract
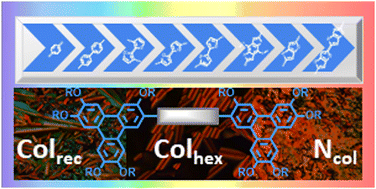
The synthesis of an exclusive family of liquid-crystalline dimers consisting of two protomesogenic triphenylene moieties connected via a rigid, π-conjugated bridge, and the study of their mesomorphous, gel self-assembly and optical properties as a function of the nature of the bridge are reported. Various triphenylene bridged dimers were successfully prepared by either palladium-catalyzed Suzuki–Miyaura cross-coupling between pentakis(alkoxy)triphenylene nonaflates and various commercially accessible aryldiboronic acids (as bridges) or by FeCl3-promoted Scholl oxidative homo-coupling of thiophene/thienothiophene/furan-containing pentakis(alkoxy)triphenylene derivatives. All linear dimers are mesomorphous, and self-organize into large multi-columnar rectangular superlattices, with columnar pairs arranged according to a chevron-like pattern, as deduced from SWAXS and supported by STM. At high temperature, most compounds also display a nematic columnar mesophase (NCol), easily recognized by characteristic optical textures. The transition temperature and mesophase ranges show a high dependency on the bridge nature and, to a lesser extent, on the chain-lengths around the triphenylene moieties. The sole kinked bridged-dimeric homolog of this series exhibits a single, room-temperature hexagonal mesophase (Colhex), as do the monofunctionalized triphenylene precursors, whereas the dimeric triphenylene with no bridge is not mesomorphous. UV-visible absorption and fluorescence emission spectra were measured in both solvents and thin films. The π-conjugated bridged-dimers exhibit emission spanning from 400–700 nm, thus covering the full visible-light range, whose emission maxima are obviously influenced by the chemical nature of the bridge. Furthermore, fluorescence quantum yields as high as 64% were measured for some of them. DFT theoretical computing calculations fully support the experimental measurements. Most dimers also form gels in cyclohexane and emit blue-to-orange light when irradiated by UV light; the corresponding xerogels revealed that their gelation ability results from the 3D morphology of entangled ultra-long and thin microfibers. The facile synthesis of these unique, multifunctional rigid discotic dimers, their rich mesomorphism, strong gelation ability and fine-tuned photophysical properties make these materials very attractive for the active field of organic electronics.
- This article is part of the themed collection: Editor’s Choice: Advances and New Avenues in Liquid Crystal Science


 Please wait while we load your content...
Please wait while we load your content...