Acridone and quinacridone derivatives with carbazole or phenoxazine substituents: synthesis, electrochemistry, photophysics and application as TADF electroluminophores†
Abstract
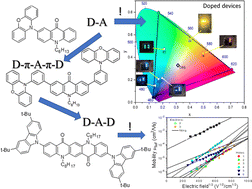
Six acridone (quinacridone) derivatives containing either carbazole or phenoxazine substituents were designed and synthesized with the aim of elucidating the effect of the donor (D) and acceptor (A) linking pattern (D–A, D–A–D or D–π–A–π–D) on their photophysical and electrochemical properties. These new electroactive compounds combine reversible electrochemical oxidation with excellent luminescent properties. Their electrochemically determined ionization potentials (IPs) are in the range from 5.09 eV to 5.45 eV, higher for derivatives with carbazole donors as compared to phenoxazine ones. The measured electron affinities (EAs) are in the range from −2.53 eV to −2.64 eV with the exception of the quinacridone derivative showing EA of −3.03 eV. Their vacuum-deposited films emit radiation in a wide spectral range from sky-blue to red. Compounds with carbazole moieties (compounds 1, 2 and 6 in the subsequent text) showed prompt fluorescence and aggregation-caused quenching. Photoluminescent quantum yields (PLQYs) of their toluene solutions reached values up to 69%. Compounds containing phenoxazine moieties (compounds marked as 3–5) demonstrated thermally activated delayed fluorescence (TADF) and aggregation-induced emission enhancement (AIEE). Their neat films showed PLQYs of 35%. Quinacridone disubstituted with carbazole (compound 6) showed the highest hole mobility reaching 2.53 × 10−3 cm2 V−1 s−1 at electric field of 3.6 × 105 V cm−1. Carbazolyl disubstituted acridone (compound 2) and phenoxazinyl monosubstituted acridone (compound 3) turned out to be ambipolar compounds showing reasonably balanced electron and hole mobilities. The appropriate combination of redox, transport and luminescent properties makes the studied compounds suitable candidates for optoelectronic applications. Test OLEDs fabricated from 3 exhibited maximum external quantum efficiencies reaching 16.7%. Finally, an excellent agreement between the experimental results and those obtained by DFT calculations should be stressed. The basics for selection according to the user needs of either D–A, D–A–D or D–π–A–π–D types of molecular structures of TADF/AIEE luminophores are provided in this study.


 Please wait while we load your content...
Please wait while we load your content...