Lithium intercalation mechanisms and critical role of multi-doping in LiFexMn2−x−yTiyO4 as high-capacity cathode material for lithium-ion batteries†
Abstract
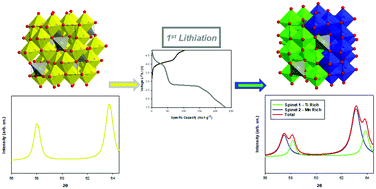
The ever-growing demand for Li-ion batteries requires high-capacity electrode materials that should also be environmentally benign, Co-free, secure and durable, to achieve an optimal compromise between sustainability and functional performances. Spinel LiMn2O4 (LMO) is a state-of-the-art material, which, in principle, could satisfy such requirements. However, an undesired cubic–tetragonal phase transition favors Jahn–Teller (J–T) spinel distortion, leading to severe capacity reduction upon cycling below 3 V. Here, we propose a novel dual-doping strategy for LMO, based on the partial substitution of Mn(III) with Fe(III) and Ti(IV) to design new active materials for high-capacity cathodes, namely LiFexMn2−x−yTiyO4 (LFMT), with Li/Mn ratio ranging between 1 and 1.7. The substitution of Mn with Fe and Ti suppresses the J–T distortion, which is often still evident in the case of Ti-doped LMO. This allows cycling in a wider voltage range (4.8–1.5 V), thus resulting in higher capacity and significantly improved stability. The lithiation mechanisms were investigated by combining ex situ X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS analyses). It demonstrated that the only redox-active metal is Mn, while Fe and Ti are electrochemically inactive. The extensive electrochemical lithiation/delithiation of the LFMT compositions brought unprecedented results, which give evidence of stabilizing cation disorder through the formation of Mn-rich and Mn-poor domains, leading to two spinel phases with different Mn:Ti ratios. These insights into the lithiation mechanism pave the way for a better understanding of the doping chemistry and electrochemistry of Mn-based spinels as cathode materials for Li-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...