Simultaneous enhancement of thermally activated delayed fluorescence and photoluminescence quantum yield via homoconjugation†
Abstract
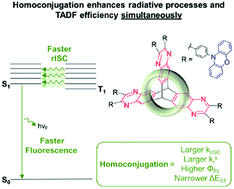
A critical challenge facing thermally activated delayed fluorescence (TADF) is to facilitate rapid and efficient electronic transitions while ensuring a narrow singlet–triplet energy gap (ΔEST) in a single luminophore. We present a TADF-active iptycene that clearly demonstrates that homoconjugation can be harnessed as a viable design strategy towards answering this challenge. A homoconjugated analogue of an established quinoxaline-based TADF luminophore has been produced by fusing three of these luminophores together across a shared triptycene core. Homoconjugation was confirmed by electrochemistry, and as a direct consequence of this phenomenon we observed synergistic improvements to photoluminescence quantum yield (ΦPL), radiative rate of singlet decay (kSr), delayed fluorescence lifetime (τTADF), and rate of reverse intersystem crossing (krISC), all while narrowing the ΔEST. The enhancement is rationalised with TD-DFT calculations including spin–orbit coupling (SOC). A facile synthesis, the ubiquity of the pyrazine motif in state-of-the-art TADF materials of all colours, and the extent of the overall performance enhancement leads to a great potential for generality.
- This article is part of the themed collection: Materials for thermally activated delayed fluorescence and/or triplet fusion upconversion


 Please wait while we load your content...
Please wait while we load your content...