Nanocrystalline BaCo3(VO4)2(OH)2 with a kagome lattice of Co(ii) ions: synthesis, crystal structure and magnetic properties†
Abstract
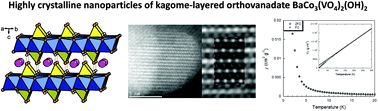
We report an initial magnetic study of highly crystalline nanoparticles of the layered-kagome compound BaCo3(VO4)2(OH)2. Quasi-spherical nanoparticles with size in the range of 9–25 nm were elaborated for the first time via a new synthetic route at ambient pressure. Rietveld refinement of X-ray diffraction data, vibrational spectroscopies and high-resolution scanning transmission electron microscopy indicates that the rhombohedral crystal structure of BaCo3(VO4)2(OH)2 is not modified by nanostructuring. Magnetisation measurements are consistent with high-spin Co2+ ions for which unquenched orbital angular momentum is present. Temperature-dependent magnetic susceptibility shows no apparent magnetic ordering or spin freezing down to 2 K and suggests finite-size or surface effects at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...