Organic–inorganic hybrid metallic conductors based on bis(ethylenedithio)tetrathiafulvalene cations and antiferromagnetic oxalate-bridged copper(ii) dinuclear anions†
Abstract
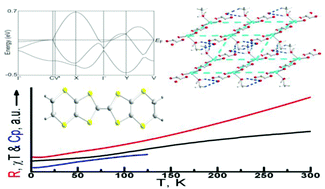
The organic–inorganic hybrid β′′-(BEDT-TTF)3[Cu2(μ–C2O4)(C2O4)2(CH3OH)(H2O)] (BEDT-TTF = bis(ethylenedithio)tetrathia-fulvalene) composed of a BEDT-TTF donor and the oxalate-bridged binuclear anion [Cu2(μ–C2O4)(C2O4)2(CH3OH)(H2O)]2− has been obtained by electrocrystallization. It crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with cell parameters of a = 7.4803(3) Å, b = 9.3547(3) Å, c = 18.6711(7) Å, α = 95.797(3)°, β = 90.974(3)°, γ = 93.508(3)°, V = 1297.06(8) Å3, and Z = 1 at 100 K. The donor arrangement belongs to the β′′ phase. From the TTF core bond lengths and Raman spectroscopy, the oxidation state of BEDT-TTF is assigned to ∼ + 2/3. CH3OH or H2O molecules bond to the metal atoms at the apical position of the square pyramid with an occupancy of 0.5. A supramolecular square lattice forms through hydrogen bonds between the antiferromagnetic binuclear anions in the anion sheet. From the band structure at 100 K, metallic conductivity is expected, which agrees with four-probe conductivity measurements: its conductivity is 11.5 S cm−1 at room temperature, increases to 160 S cm−1 at 7.6 K, and then decreases to 150 S cm−1 at 2 K. From magnetic measurements, there is no long-range magnetic ordering, which is confirmed by specific heat measurements.
space group with cell parameters of a = 7.4803(3) Å, b = 9.3547(3) Å, c = 18.6711(7) Å, α = 95.797(3)°, β = 90.974(3)°, γ = 93.508(3)°, V = 1297.06(8) Å3, and Z = 1 at 100 K. The donor arrangement belongs to the β′′ phase. From the TTF core bond lengths and Raman spectroscopy, the oxidation state of BEDT-TTF is assigned to ∼ + 2/3. CH3OH or H2O molecules bond to the metal atoms at the apical position of the square pyramid with an occupancy of 0.5. A supramolecular square lattice forms through hydrogen bonds between the antiferromagnetic binuclear anions in the anion sheet. From the band structure at 100 K, metallic conductivity is expected, which agrees with four-probe conductivity measurements: its conductivity is 11.5 S cm−1 at room temperature, increases to 160 S cm−1 at 7.6 K, and then decreases to 150 S cm−1 at 2 K. From magnetic measurements, there is no long-range magnetic ordering, which is confirmed by specific heat measurements.
- This article is part of the themed collection: Special issue in honour of Daoben Zhu


 Please wait while we load your content...
Please wait while we load your content...