A novel self-healing hydrogel based on derivatives of natural α-amino acids with potential applications as a strain sensor
Abstract
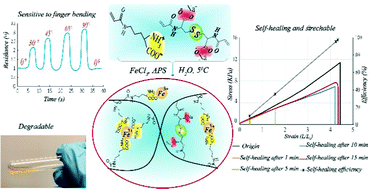
We successfully synthesized a novel hydrogel based on derivatives of natural α-amino acids: ornithine and cystine. To make ornithine attachable to the polymer chain, the δ-amino group was modified with an acryloyl group and the main monomer was obtained. From cystine, the cross-linker N,N′-bisacryloylcystine was obtained. Then, by free radical polymerization of the monomers in the presence of Fe3+, the hydrogel was obtained. The presence of iron ions in the pre-gel solution accelerated the decomposition of a free radical initiator (ammonium persulfate) and allowed uniform distribution of complexed Fe3+ in the hydrogel to be obtained. The presence of free α-amino acid groups in the main monomer and then in the polymeric network of the gels enables this complexation. As a result, the obtained hydrogel benefits from the chemical and physical cross-links, disulfide bonds, and metal–ligand complex, respectively. The composition of the hydrogel was optimized to obtain improved mechanical properties and self-healing ability. Thereby we identified a hydrogel exhibiting fast and conclusive self-healing, which recovered approximately 99% (efficiency of self-healing based on fracture strain) of its original properties after 15 min. The conductivity and electrical response of the hydrogel were investigated. The results revealed a rapid electrical response to minor stretching of the hydrogel, allowing it to be used as a strain sensor. In addition, the presence of the disulfide bonds in the hydrogel structure enabled the hydrogel to degrade in a redox environment.


 Please wait while we load your content...
Please wait while we load your content...