Inverting glucuronidation of hymecromone in situ by catalytic nanocompartments†
Abstract
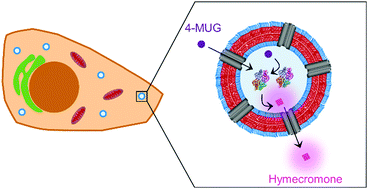
Glucuronidation is a metabolic pathway that inactivates many drugs including hymecromone. Adverse effects of glucuronide metabolites include a reduction of half-life circulation times and rapid elimination from the body. Herein, we developed synthetic catalytic nanocompartments able to cleave the glucuronide moiety from the metabolized form of hymecromone in order to convert it to the active drug. By shielding enzymes from their surroundings, catalytic nanocompartments favor prolonged activity and lower immunogenicity as key aspects to improve the therapeutic solution. The catalytic nanocompartments (CNCs) consist of self-assembled poly(dimethylsiloxane)-block-poly(2-methyl-2-oxazoline) diblock copolymer polymersomes encapsulating β-glucuronidase. Insertion of melittin in the synthetic membrane of these polymersomes provided pores for the diffusion of the hydrophilic hymecromone–glucuronide conjugate to the compartment inside where the encapsulated β-glucuronidase catalyzed its conversion to hymecromone. Our system successfully produced hymecromone from its glucuronide conjugate in both phosphate buffered solution and cell culture medium. CNCs were non-cytotoxic when incubated with HepG2 cells. After being taken up by cells, CNCs produced the drug in situ over 24 hours. Such catalytic platforms, which locally revert a drug metabolite into its active form, open new avenues in the design of therapeutics that aim at prolonging the residence time of a drug.
- This article is part of the themed collection: 2022 Journal of Materials Chemistry B Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...