Synthesis and photophysical properties of a new push–pull pyrene dye with green-to-far-red emission and its application to human cellular and skin tissue imaging†
Abstract
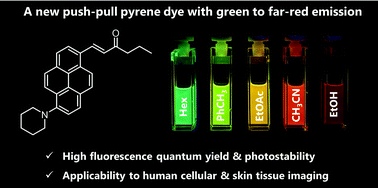
Herein, we discuss a new pyrene-based push–pull dye (PC) and our investigation of its photophysical properties and applicability to biological studies. The newly synthesized dye exhibits highly polarity-sensitive fluorescence over a significantly wide range (i.e., the green to far-red region), accompanied by high fluorescence quantum yields (ΦFL > 0.70 in most organic solvents) and superior photostability to that of the commonly used Nile Red (NR) dye, which also fluoresces in the green to red region. When human prostate cancer cells stained with PC were imaged using a confocal laser scanning fluorescence microscope, PC was found to selectively stain the lipid droplets. Under the cell conditions where the formation of droplets was inhibited, PC could be distributed to both the remaining droplets and the intercellular membranes, which could be distinguished based on the fluorescence solvatochromic function of PC. Furthermore, PC efficiently stained normal human skin tissue blocks treated with a transparency-enhancing agent and enabled clear visualization of individual cells in each tissue architecture by means of two-photon fluorescence microscopy (2PM). Interestingly, PC provides bright 2PM images under tissue-penetrative 960 nm excitation, realizing much clearer and deeper tissue imaging than conventional pyrene dyes and NR. These results suggest that PC could replace several commonly used dyes in various biological applications, particularly the rapid and accurate diagnosis of tissue diseases, typified by biopsy.


 Please wait while we load your content...
Please wait while we load your content...