Thioredoxin reductase-triggered fluorogenic donor of hydrogen sulfide: a model study with a symmetrical organopolysulfide probe with turn-on near-infrared fluorescent emission†
Abstract
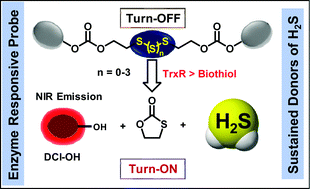
We describe herein the rational development of an organopolysulfide-based fluorogenic donor of hydrogen sulfide (H2S) DCI-PS, which can be activated by the antioxidant selenoenzyme thioredoxin reductase (TrxR) with concomitant release of the dicyanoisophorone-based near-infrared (NIR) fluorophore. Along with the polysulfide probe DCI-PS capable of releasing the NIR fluorophore and H2S, the corresponding disulfide-probe DCI-DS was also rationally designed and synthesized, which releases the fluorophore without donating H2S. Detailed spectroscopic and kinetic studies in an aqueous medium revealed significantly higher reactivity of the probes towards DTT (for TrxR activity) over the well-known cellular abundant biothiol GSH. Mechanistically, the nucleophilic attack at the disulfide/polysulfide linkage by the thiol/selenol group of the bio-analytes leads to the self-immolative cyclization process with the release of the turn-on fluorophore with/without H2S. Considering the overexpression of mammalian TrxR in cancer cells, the turn-on fluorogenic H2S donation process from the cellular non-toxic DCI-PS was validated in a representative breast cancer cell line (MDA-MB-231) for the sustained donation of H2S with concomitant release of the red-emitting NIR fluorophore. The TrxR-triggered fluorescence turn-on process in DCI-PS was further supported by the significant inhibition of the fluorogenic process in the presence of TrxR-selective small-molecule inhibitors and by the significant binding affinity predicted by the protein–ligand docking study. Results with the antioxidant enzyme-triggered intracellular sustained donation of H2S with concomitant fluorescence turn-on will certainly find wider biomedical applications in the near future, particularly in H2S-mediated therapeutics in disease states.


 Please wait while we load your content...
Please wait while we load your content...