Catalytic centers with multiple oxidation states: a strategy for breaking the overpotential ceiling from the linear scaling relation in oxygen evolution†
Abstract
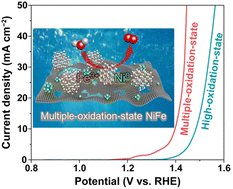
Transition metals with high oxidation states are considered the primary catalytically active sites for the oxygen evolution reaction (OER) with high intrinsic reactivity. However, their kinetics and energetics require further development to achieve an efficient OER performance. Herein, we report a facile strategy for modulating the OER kinetics and energetics of high-oxidation-state active metals by incorporating a low-oxidation-state transition metal. A synthesized multiple-oxidation-state NiFe catalyst consisting of high-oxidation-state Fe3+ and low-oxidation-state Ni0 exhibited excellent OER activity with a low overpotential of 160 mV at 10 mA cm−2, surpassing the performance of OER catalysts that consist only of high- or low-oxidation-state active metals. Through mechanistic studies, we confirmed that the introduction of Ni0 accelerated the charge transfer kinetics and decreased the energy barrier for water oxidation. This study presents a new perspective on the modification of the oxidation state of the active metal for optimizing the OER process.


 Please wait while we load your content...
Please wait while we load your content...