Topotactic BI3-assisted borodization: synthesis and electrocatalysis applications of transition metal borides†
Abstract
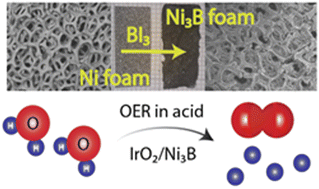
A facile and universal route for synthesizing transition metal borides has been developed by reaction of boron triiodide (BI3) with elemental transition metals. This method employs relatively low synthesis temperatures to afford single-phase samples of various binary and ternary metal borides, such as Fe2B, Co2B, Ni3B, TiB2, VB2, CrB2, and Ni2CoB. This synthesis protocol can be utilized for the topotactic transformation of metal shapes into their respective borides, as exemplified by transformation of Ni foam to Ni3B foam. In situ powder X-ray diffraction studies of the Ni–BI3 system showed that the crystalline nickel borides, Ni4B3 and Ni2B, start to form at temperatures as low as 700 K and 877 K, respectively, which is significantly lower than the typical synthesis temperatures required to produce these borides. Ni3B synthesized by this method was tested as a supporting material for oxygen evolution reaction (OER) in acidic media. Composite electrocatalysts of IrO2/Ni3B with only 50% of IrO2 exhibit current densities and stability similar to pure IrO2 at mass loadings lower than 0.5 mg cm−2, indicating Ni3B could be a promising supporting material for acidic OER.


 Please wait while we load your content...
Please wait while we load your content...