Developing a nitrile-based lithium-conducting electrolyte for low temperature operation†
Abstract
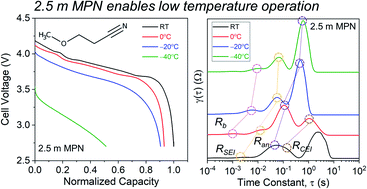
Lithium-ion (Li+) batteries are considered the most attractive for low temperature operation. Though Li+-conducting electrolytes predominately use carbonate solvents, we show that nitriles, such as 3-methoxypropyionitrile (MPN), are promising candidates for use at low temperatures. At a high salt concentration (2.5 molal), and combined with a fluoroethylene carbonate additive, this electrolyte enables −40 °C operation when configured in a graphite‖lithium cobalt oxide cell. We leverage molecular dynamics simulations and experimentally validate Li+ diffusivity/conductivity measurements to bolster our understanding of the MPN electrolyte in comparison with carbonates. At room temperature, cells demonstrate high rate capability (100 mA h g−1 discharge capacity at 2C), and also maintain >75% of their initial capacity up to 100 cycles when cycled at 0.2C. At −40 °C, >50% of the cell's room temperature discharge capacity is sustained, showing exemplary low temperature performance. By performing an impedance-based distribution of relaxation times analysis, we identify that interfacial kinetics at the anode surface and the cathode electrolyte interphase are the two underlying factors limiting low temperature operation. The results presented herein offer an exciting direction for the discovery and implementation of nitrile-based solvents that can withstand low temperatures.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...