Reaction mechanism and kinetics for carbon dioxide reduction on iron–nickel Bi-atom catalysts†
Abstract
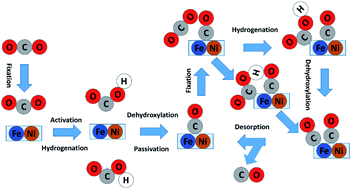
The electrochemical CO2 reduction reaction (CO2RR) is being accepted as one of the most promising strategies to convert carbon emissions to valuable chemicals and fuels. Among the various types of electrocatalysts, dual-site catalysts have emerged as a new frontier. In particular, the Ni–Fe bi-site not only plays a key role in the biological utilization of CO2 in enzymes but also shows exceptional activity and selectivity for CO evolution in the heterogeneous CO2RR. However, an in-depth understanding of the reaction mechanism has not been achieved. In this work, we applied the recently developed grand canonical potential kinetics (GCP-K) method to determine the reaction mechanism and kinetics of the CO2RR on Fe&Ni@g-N6. Unlike the traditional CO2RR mechanism on transition metals or single-atom catalysts, the Fe–Ni dual site is firstly passivated by strongly adsorbed *CO, and the real active site of the CO2RR cycle is at the exposed single Fe site. The predicted overall kinetics has a Uonset (10 mA cm−2) of −0.99 V to generate CO, and the maximum turnover frequency reaches 961 h−1 per site at U = −1.18 V. The competitive hydrogen evolution reaction (HER) has a greater negative onset potential and does not interfere with the CO2RR. These predictions show a reasonable agreement with the experiment. The promising performance is correlated with the down-shift and localization of the 3d electronic states of Fe atoms. Our analysis provides a defined mechanism and offers useful insight into designing efficient dual-active-site electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...