Li nucleation on the graphite anode under potential control in Li-ion batteries†
Abstract
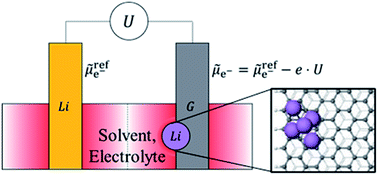
Application of Li-ion batteries in electric vehicles requires improved safety, increased lifetime and high charging rates. One of the most commonly used intercalation anode material for Li-ion batteries, graphite, is vulnerable to Li nucleation, a side reaction which competes with the intercalation process and leads to loss of reversible capacity of the battery, ageing and short-circuits. In this study, we deploy a combined grand canonical large-scale electronic density-functional theory (DFT) and Poisson–Boltzmann electrolyte theory to study the nucleation and growth of Li clusters on the graphite anode in the presence of its surrounding electrolyte environment at different applied voltages with respect to the Li metal reference electrode. We find the voltage below which the nucleation energy becomes negative (corresponding to Li nucleation becoming energetically favourable), the ‘potential of zero nucleation energy’ (UPZN). We observe a distinct minimum in the plots of UPZN as a function of the size of nucleated clusters. When the applied voltage on the graphite electrode is below the minimum value of UPZN, the nucleated clusters start growing unbounded on graphite electrode. This potential for cluster growth (UPCG) is found to be −0.12 V on the periodic basal plane of unlithiated graphite and −0.08 V on lithiated graphite. The corresponding potential for the zigzag edge termination is −0.06 V on unlithiated graphite and −0.04 V on lithiated graphite. Thus, the nucleation and cluster growth is favored on the zigzag edge termination of the graphite electrode as compared to the periodic basal plane and on the lithiated graphite as compared to the unlithiated graphite. We find that the surrounding environment plays a significant role and that nucleation is more likely to occur in electrolyte environment than that predicted from calculations in vacuum. We observe that the potentials obtained with grand canonical ensemble DFT method in electrolyte are close to experimentally available data. The study has profound implications for the nucleation, growth and control of metal dendrites in a battery cell.


 Please wait while we load your content...
Please wait while we load your content...