Tuning the interfacial chemistry for stable and high energy density aqueous sodium-ion/sulfur batteries†
Abstract
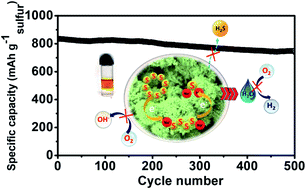
Low cost, highly safe, and environmentally benign aqueous rechargeable sodium-ion batteries (ARSIBs) are promising energy storage devices for the future. However, low cell voltage, low energy density, and inadequate cycling stability associated with low-capacity electrode materials, the restricted water stability window (1.23 V), and the inability of aqueous electrolyte to form a stable solid electrolyte interphase (SEI) is a major concern. In the present work, we have designed an aqueous rechargeable Na-ion/S battery, using a S@CoWO4 anode coupled with a Na–W–U–D electrolyte prepared by mixing NaClO4, urea, and N,N-dimethylformamide (DMF) in water, that exhibits a remarkable stability window of 3.1 V due to reduced water activity and stable SEI formation. The excellent anchoring ability and accelerated polysulfide redox kinetics of CoWO4 coupled with the high stability of Na–W–U–D electrolyte results in a very high capacity of 834 mA h g−1 (w.r.t sulfur) at 0.5C with 88% retention after 500 cycles. Post stability XPS and SEM studies provide evidence of a smooth and stable SEI consisting of Na2CO3, polyurea, and reduced products of DMF, which prevent polysulfide dissolution and side reactions due to water electrolysis electrolyte evaporation and dissolved oxygen. Further, a full cell assembled by integrating the S@CoWO4 anode and Na0.44MnO2 cathode in the Na–W–U–D electrolyte shows remarkable durability and delivers an energy density of 119.0 W h kg−1, demonstrating the excellent potential of the cell for scale-up and manufacturing.


 Please wait while we load your content...
Please wait while we load your content...