In situ reconstruction enhanced dual-site catalysis towards nitrate electroreduction to ammonia†
Abstract
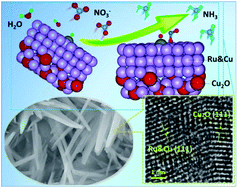
Electro-reduction of nitrate to ammonia (e-NRA) has been considered as a facile and promising approach to eliminate nitrate pollution and produce ammonia (NH3) under ambient conditions. Nevertheless, in recent state-of-the-art studies, high faradaic efficiency towards NH3 (FENH3) was usually achieved with a very negative working potential (vs. RHE) or high overpotential, which causes not only low cost-efficiency but also structural degradation in electrocatalysts. In this work, we utilize the strategy of in situ electrochemical reconstruction to develop a highly efficient and durable electrocatalyst Ru&Cu/Cu2O (denoted as i-Cu5Ru1Ox) towards alkaline e-NRA. As a result, highly selective production of NH3 can be achieved with an optimal FENH3 of around 95% even at a positive working potential of 0.1 V (vs. RHE) or a small overpotential of 0.59 V. The catalyst shows no obvious degradation after consecutive e-NRA for 10 h. In addition, density functional theory (DFT) computations reveal that the excellent catalytic performance of Ru&Cu/Cu2O could be attributed to the synergy of the Cu/Ru dual-site, which results in significantly enhanced adsorption of NO3− ion and a more favorable proton supply for the hydrogenation during e-NRA. This work thus highlights the importance of dual-site synergy towards the electrochemical process with multiple elementary steps.


 Please wait while we load your content...
Please wait while we load your content...