Heterointerfaces of nickel sulphides and selenides on Ni-foam as efficient bifunctional electrocatalysts in acidic environments†
Abstract
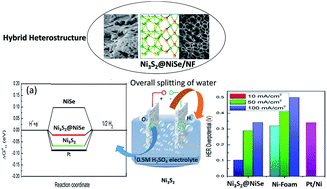
The fulfilment of simultaneous hydrogen and oxygen evolution reactions (HER and OER) in acidic conditions is one of the challenges facing the production of green hydrogen. Herein, robust electrocatalysts using a heterostructure of Ni-sulphide (NiS, NiS2 and Ni3S2) and -selenide (NiSe) supported on Ni-foam (NF) have been developed via the simple single-step thermal diffusion of S and Se. Among the various prepared hierarchical structures, Ni3S2@NiSe/NF shows the best catalytic activity for the HER, with low overpotentials of 103 and 289 mV at current densities of 10 and 50 mA cm−2, respectively. It shows a promising Tafel slope of 74.2 mV dec−1 in 0.5 M H2SO4 for the HER. The same structure also shows remarkable OER activity in acidic conditions, with an overpotential of 0.26 V (vs. RHE) at 50 mA cm−2 and a Tafel slope of 68.9 mV dec−1. The density functional theory (DFT)-based approach reveals that the Ni3S2@NiSe heterointerface exhibits a very low adsorption free energy for hydrogen at the cathode and strong electron localization across its interface, which facilitates the charge-transfer kinetics at the anode to improve the sluggish OER rate. In addition, its excellent HER performance results from the strong hybridization of the Ni d orbitals with the S and Se p orbitals, destabilizing their antibonding characteristics.


 Please wait while we load your content...
Please wait while we load your content...