Strong interfacial energetics between catalysts and current collectors in aqueous sodium–air batteries†
Abstract
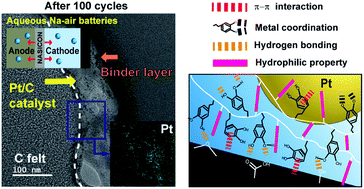
Conventional binders, such as polyvinylidene fluoride, are not ideal candidates for aqueous sodium–air batteries (SABs) because of their relatively low adhesiveness, weak mechanical strength, and inherent hydrophobicity. The low adhesion strength often leads to electrocatalysts and carbon current collector detachments, followed by degradation of electrochemical performance over time. For SABs to possess excellent performance, development of advanced polymeric binders with high wettability and underwater adhesion is required. In this study, the adhesion stability of the electrocatalyst/current collector interface is significantly enhanced by using synthesized catechol-derivative hydrophilic binders, whereas catalyst desorption and carbon corrosion are effectively prevented. Using high-resolution transmission electron microscopy, we demonstrated the role of synthesized binders on the morphological stability of the composite electrode. Furthermore, surface forces apparatus and density functional theory calculations reveal insights into how polymeric binders impact the adhesion mechanism and battery performance of SAB based on their functional groups.


 Please wait while we load your content...
Please wait while we load your content...