Variable-valence ion and heterointerface accelerated electron transfer kinetics of electrochemical water splitting†
Abstract
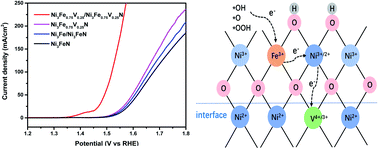
Accelerating electron transfer kinetics is an efficient strategy to tackle the sluggish oxygen evolution reaction (OER). Herein, Ni3Fe1−xVx/Ni3Fe1−xVxN heterojunctions were elaborately constructed to demonstrate that the coupling of variable-valence metal doping and nanoalloy/nitride heterointerfaces could optimize the OER performance of antiperovskite nitrides. The spectroscopic results suggested that during OER electrochemical surface reconstruction occurred to form an assembly of a crystalline Ni3Fe1−xVx/Ni3Fe1−xVxN heterojunction core and amorphous NiFeOOH shell (Ni3Fe1−xVx/Ni3Fe1−xVxN@NiFeOOH). The electron transfer from the OER intermediates via the amorphous NiFeOOH shell was efficiently accelerated by the variable-valence V3+/4+ electron acceptor at the core@shell interface and the heterojunction effect in the Ni3Fe1−xVx/Ni3Fe1−xVxN core. As a result, the oxygen and hydrogen evolution reactions can occur at low overpotentials of 260 mV at 50 mA cm−2 and 113 mV at 10 mA cm−2, respectively, affording a low cell voltage of 1.66 V at 10 mA cm−2 for water splitting in alkaline electrolyte (1.0 M KOH). Our results provide a new attempt at improving water splitting kinetics via the variable-valence ion coupling heterojunction effect.


 Please wait while we load your content...
Please wait while we load your content...