Lanthanide metal–organic frameworks for the fixation of CO2 under aqueous-rich and mixed-gas conditions†
Abstract
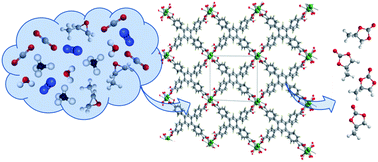
The development of effective catalysts is one of the big challenges associated with a new circular carbon economy addressing climate change. The targeted synthesis of robust and recyclable catalysts can be made possible through elucidation of the molecular interactions between the active sites in metal–organic frameworks (MOFs) with potentially reactive molecules. Herein, we present the use of lanthanide (Ln)-based MOFs – [Ln(HTCPB)] Ln: Ce, Nd, Sm, Eu, Tb, and Dy; H4TCPB: 1,2,4,5-tetrakis(4-carboxyphenyl)benzene – as catalysts for the conversion of CO2 to value-added products. Using these MOFs, we describe the fixation of CO2 into the epoxy ring of propylene oxide for the production of cyclic carbonates. Structural analysis of the propylene oxide (PO)-loaded MOF revealed the binding of PO to Ce3+, confirming the key role of open Ce3+ sites in reducing the activation energy of the PO chemical transformation. The ring-opening of the PO molecule is followed by the insertion of one molecule of CO2, leading to the formation of the propylene carbonate (PC) molecule. The channels present in the MOF structure allow for diffusion of CO2 from the environment to the open Ce3+ sites and that of PC in the opposite direction, thus upholding the catalytic process. [Ce(HTCPB)] exhibited high catalytic activity toward PC production with a turn-over frequency (TOF) = 20.2 h−1, one of the highest reported. The catalyst showed no catalytic deterioration after three cycles and maintained a comparable catalytic activity when aqueous-rich conditions and gas CO2/N2 and CO2/CH4 mixtures were used, highlighting that non-contributing gas molecules or impurities do not affect the catalytic activity of the MOF. Catalytic experiments using [Ln(HTCPB)] demonstrated that the rate of CO2 conversion decreased by decreasing the ionic radii of the Ln used. Grand canonical Monte Carlo simulations (GCMC) and topographic steric maps suggested that the activity of a catalyst can be tuned by manipulating the ionic size and partial charge of the lanthanides, and steric properties of the active site. Our study highlights the engineering of efficient catalysts capable of converting CO2 into value-added products directly from its wet and mixed-composition streams (e.g., waste flue gas and biogas).


 Please wait while we load your content...
Please wait while we load your content...