Structural, electronic, and electrocatalytic evaluation of spinel transition metal sulfide supported reduced graphene oxide†
Abstract
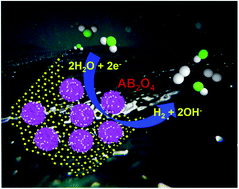
Development of highly active and durable non-precious spinel transition metal sulfide (STMS)-based electrocatalysts plays a vital role in increasing the efficiency of hydrogen production via water electrolysis. Herein, we have synthesized a hierarchical nanostructured ZnCo2S4 on reduced graphene oxide (ZCS@rGO) sheet using a cost-effective hydrothermal synthesis method. The prepared ZCS@rGO shows improved hydrogen desorption and adsorption energy of the electrocatalyst surface towards efficient hydrogen evolution reaction (HER). As a result, ZCS@rGO showed lower HER overpotential (η10 = 135 eV) and Tafel slope (47 mV dec−1) and superior durability at 10 mA cm−2 for 36 h, as compared to the benchmark catalyst of Pt–C. Further, the electronic structure and HER mechanism of the ZCS@rGO catalyst were investigated by density functional theory calculations. This work provides a new pathway for the rational design of highly active and durable non-precious STMS-based electrocatalysts for hydrogen production.
- This article is part of the themed collection: 2023 Journal of Materials Chemistry A Lunar New Year collection


 Please wait while we load your content...
Please wait while we load your content...