Hierarchical assembly of pH-responsive surfactant–cyclodextrin complexes†
Abstract
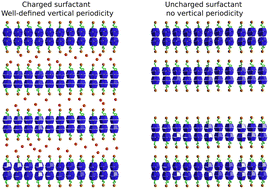
In this work, the inclusion complexes of alkyl ethoxy carboxylates with α-cyclodextrin (αCD) and β-cyclodextrin (βCD) were investigated. The thermodynamics of the complexation process was probed by isothermal titration calorimetry (ITC) and volumetry as a function of the degree of ionization of the surfactant. The complexation process was shown to be an enthalpically driven pH-independent process. For both types of cyclodextrins, the complexes were found to spontaneously self-assemble into highly-ordered supramolecular aggregates probed by small-angle neutron scattering and electron and optical microscopy. Herein, we report the formation of thin platelets for nonionized surfactant systems and equally spaced multilayered hollow cylinders for ionized systems in a hierarchical self-assembly process. In addition, the analysis allowed unveiling the effect of the number of ethylene oxides in the surfactants and the CD cavity size on the morphology of the aggregates. Finally, this study also highlights the importance of examining the tuning parameters' influence on the short and long-range interactions involved in the control of the assembly process.


 Please wait while we load your content...
Please wait while we load your content...