Subtle changes in pH affect the packing and robustness of fatty acid bilayers†
Abstract
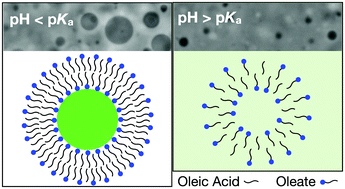
Connecting molecular interactions to emergent properties is a goal of physical chemistry, self-assembly, and soft matter science. We show that for fatty acid bilayers, vesicle rupture tension, and permeability to water and ions are coupled to pH via alterations to lipid packing. A change in pH of one, for example, can halve the rupture tension of oleic acid membranes, an effect that is comparable to increasing lipid unsaturation in phospholipid systems. We use both experiments and molecular dynamics simulations to reveal that a subtle increase in pH can lead to increased water penetration, ion permeability, pore formation rates, and membrane disorder. For changes in membrane water content, oleic acid membranes appear to be more than a million times more sensitive to protons than to sodium ions. The work has implications for systems in which fatty acids are likely to be found, for example in the primitive cells on early Earth, biological membranes especially during digestion, and other biomaterials.
- This article is part of the themed collection: Soft Matter Emerging Investigators Series


 Please wait while we load your content...
Please wait while we load your content...