Experimental and molecular dynamics studies on aggregation behaviour of salicylaldehyde azine ester†
Abstract
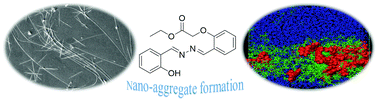
Aggregation phenomena arise predominantly due to self-organisation of molecules to form supramolecular assemblies leading to restriction of intramolecular motions. In the present study, the solvent-induced aggregation of salicylaldehyde azine ester (SAE) was comprehensively investigated through experimental techniques, and classical molecular dynamics simulations (MDS). The emission spectra and particle sizes of SAE in THF–water mixtures confirmed the formation of nanoaggregates. The interaction of SAE aggregates with the solvent mixture was studied using Fourier-transform Infrared spectroscopy. The optical microscopy images and surface morphology analysis reinforced the nanoaggregate formation of SAE in solvent mixtures with increasing water fractions. The average number of H-bonds, diffusion coefficients and trajectory density contours of the aggregates were investigated through MDS studies, which provided atomistic perceptions into the formation of rod-like SAE nanoaggregates. The combined results of experimental and theoretical studies offer deeper insights into the self-aligning tendency of SAE in THF–water mixtures.


 Please wait while we load your content...
Please wait while we load your content...