Kinetics of geopolymerization followed by rheology: a general model
Abstract
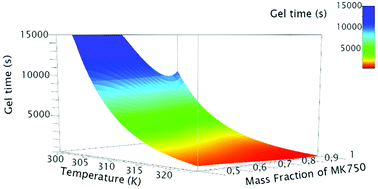
The geopolymerization process necessitates the activation of an aluminosilicate source by an alkaline solution. Its kinetics is followed by rheology. The storage modulus (G′) and loss modulus (G′′) are monitored through oscillatory rheological measurements from the early stage, geopolymer paste, to the gel point with the formation of a geopolymer network. The results show that the temperature increase shortens the reaction time. The principle of Reaction Time–Temperature Superposition (RTTS) is introduced to predict this phenomenon. Furthermore, it is pointed out that using metakaolin blends with different reactivities allows modifying and controlling the reaction time. A critical weight fraction of reactive metakaolin is identified as necessary for the formation of the geopolymer network. The reaction times of the different formulations are linked to the temperature and the weight fraction of metakaolin by the Arrhenius law. A model is established to predict the reaction time according to the temperature and the weight fraction between the two metakaolins used.


 Please wait while we load your content...
Please wait while we load your content...