Improved water oxidation activity of a Sillén SrBi3O4Cl3 photocatalyst by flux method with an appropriate binary-component molten salt†
Abstract
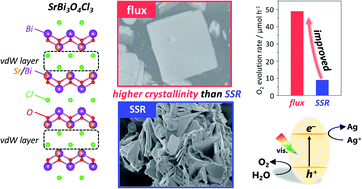
A series of Bi-based Sillén(–Aurivillius)-layered oxyhalides has recently attracted considerable attention as promising photocatalysts for visible-light water splitting owing to their suitable band levels and high durability, both of which are derived from their unique band structures. In this study, we investigate a Sillén-type visible-light-responsive SrBi3O4Cl3, which consists of fluorite and single/double halogen layers, as an O2-evolving photocatalyst, with interest in double halogen layers (van der Waals (vdW) layers). To obtain highly crystalline SrBi3O4Cl3 particles for improving photocatalytic activity, we focus on flux synthesis using various chloride salts as flux agents. We reveal that only a specific binary-component molten salt, KCl/SrCl2, provides highly crystalline and plate-like SrBi3O4Cl3 particles with a pure phase, whereas the others fail. This specificity of KCl/SrCl2 is due to the appropriate ionic radius of K+ and the specific phase diagram of KCl/SrCl2, both of which are associated with the intercalation of metal cations into the vdW layers during the flux synthesis. With an increase in the calcination temperature from 873 to 973 K, the flux synthesis enhances the crystallinity of the SrBi3O4Cl3 particles with suppressed Cl defect formation, whereas the conventional solid-state reaction (SSR) synthesis significantly increases the amount of Cl defects because of volatilization. Therefore, the nanoplate-like particles obtained by the flux method have relatively high crystallinity, thereby exhibiting considerably better charge carrier dynamics and higher photocatalytic activity than those obtained by SSR.


 Please wait while we load your content...
Please wait while we load your content...