Bioderived ether design for low soot emission and high reactivity transport fuels†
Abstract
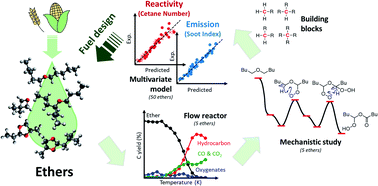
Bioderived ethers have recently drawn attention as a response to increasing demands for clean alternative fuels. A theory-experiment combined approach was introduced for five ether molecules representing linear, branched, and cyclic ethers to derive rational design principles for low-emission and high-reactivity ethers. Flow reactor experiments and quantum-mechanical calculations were performed at high- (750–1100 K) and low-temperature (400–700 K) regimes to investigate the structural effects on their sooting tendency and reactivity, respectively. At high-temperatures, the high-sooting tendency of ethers is related to increased C3 and C4 hydrocarbon formation compared to C1 and C2 products from oxidation reactions. On the other hand, the reactivity in the low-temperature regime is determined by the activation energies of reaction steps leading to ketohydroperoxide formation. These studies found that the sooting tendency and reactivity of ethers are relevant to two structural factors: the carbon type (primary to quaternary) and the relative position of ether oxygen atoms to carbon atoms. These factors were utilized to build a multivariate regression model, fitted to the cetane number (CN) and yield sooting index (YSI) of 50 different ethers. The model suggests building blocks with specific carbon types that maximize the CN and minimize the YSI, leading to design principles for ethers having low-emissions and high-reactivity as fuels for transport applications. Ethers with a high CN and low YSI were then proposed using the developed model, and through experimental measurements, it was demonstrated that they are promising biodiesel candidates.


 Please wait while we load your content...
Please wait while we load your content...