Chemical reduction of Prussian blue nanocubes to obtain alkali ion containing cathodes and their battery applications†
Abstract
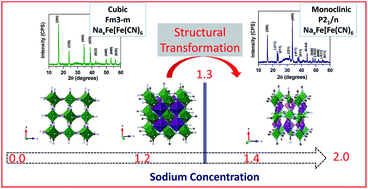
A facile process for the preparation of alkali ion containing Prussian blue cathodes by chemical reduction and their battery applications are reported. Two cathodes, LixFe[Fe(CN)6] and NaxFe[Fe(CN)6], have been prepared through chemical lithiation/sodiation of Fe[Fe(CN6)] through a rapid (<10 min) and low temperature process (<100 °C). Structural analysis confirmed that the as-prepared Fe[Fe(CN)6], lithiated and sodiated compounds maintained a cubic structure. Nano-sized cuboidal morphology was confirmed by electron microscopy and it was unaffected by the chemical lithiation/sodiation process. Based on the first cycle electrochemical capacity, it is realized that chemical sodiation into Fe[Fe(CN6)] is more efficient than lithiation. The sodiated Fe[Fe(CN)6] showed a capacity retention of about 70% after 500 cycles with 78 mA h g−1 discharge capacity at 100 mA g−1 for sodium ion battery applications. On the other hand, lithiated Fe[Fe(CN)6] showed a better capacity retention of 92% after 300 cycles with 111 mA h g−1 discharge capacity at 100 mA g−1 for lithium ion battery applications. Ex situ X-ray diffraction analysis of the electrodes at the end of the first charge, at the end of the first cycle and at the end of 5 cycles was carried out and the results were compared with those of the as-sodiated NaxFe[Fe(CN)6] electrode. This investigation confirmed that for the chemically sodiated electrode a cubic phase at the end of the first charge transformed to a monoclinic structure at the end of discharge (first cycle and at the end of the 5th cycle). The cubic to monoclinic structural phase transition is identified to be happening at the threshold sodium concentration around x = 1.3 in NaxFe[Fe(CN)6]. These results are correlated with ex situ surface chemical analysis. Such a cost-effective chemical reduction process to achieve a sodium-rich phase has not been reported so far to the best of our knowledge.


 Please wait while we load your content...
Please wait while we load your content...