Catalytic photochemical enantioselective α-alkylation with pyridinium salts†
Abstract
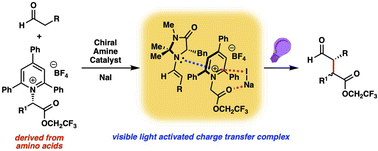
We have developed a chiral amine catalyzed enantioselective α-alkylation of aldehydes with amino acid derived pyridinium salts as alkylating reagents. The reaction proceeds in the presence of visible light and in the absence of a photocatalyst via a light activated charge-transfer complex. We apply this photochemical stereoconvergent process to the total synthesis of the lignan natural products (−)-enterolactone and (−)-enterodiol. Mechanistic studies support the ground-state complexation of the reactive components followed by divergent charge-transfer processes involving catalyst-controlled radical chain and in-cage radical combination steps.


 Please wait while we load your content...
Please wait while we load your content...