Solvent-controlled ion-coupled charge transport in microporous metal chalcogenides†
Abstract
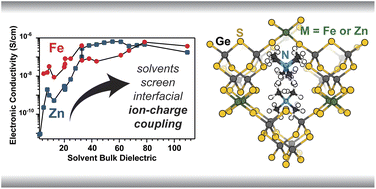
Interactions between ions and itinerant charges govern electronic processes ranging from the redox chemistry of molecules to the conductivity of organic semiconductors, but remain an open frontier in the study of microporous materials. These interactions may strongly influence the electronic behavior of microporous materials that confine ions and charges to length scales comparable to proton-coupled electron transfer. Yet despite mounting evidence that both solvent and electrolyte influence charge transport through ion–charge interactions in metal–organic frameworks, fundamental microscopic insights are only just beginning to emerge. Here, through electrochemical analysis of two open-framework chalcogenides TMA2FeGe4S10 and TMA2ZnGe4S10, we outline the key signatures of ion-coupled charge transport in band-type and hopping-type microporous conductors. Pressed-pellet direct-current and impedance techniques reveal that solvent enhances the conductivity of both materials, but for distinct mechanistic reasons. This analysis required the development of a fitting method that provides a novel quantitative metric of concerted ion–charge motion. Taken together, these results provide chemical parameters for a general understanding of electrochemistry in nanoconfined spaces and for designing microporous conductors and electrochemical methods used to evaluate them.
- This article is part of the themed collection: Energy Frontiers: Electrochemistry and Electrochemical Engineering


 Please wait while we load your content...
Please wait while we load your content...