Coordination-induced bond weakening of water at the surface of an oxygen-deficient polyoxovanadate cluster†
Abstract
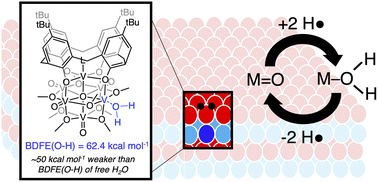
Hydrogen-atom (H-atom) transfer at the surface of heterogeneous metal oxides has received significant attention owing to its relevance in energy conversion and storage processes. Here, we present the synthesis and characterization of an organofunctionalized polyoxovanadate cluster, (calix)V6O5(OH2)(OMe)8 (calix = 4-tert-butylcalix[4]arene). Through a series of equilibrium studies, we establish the BDFE(O–H)avg of the aquo ligand as 62.4 ± 0.2 kcal mol−1, indicating substantial bond weaking of water upon coordination to the cluster surface. Subsequent kinetic isotope effect studies and Eyring analysis indicate the mechanism by which the hydrogenation of organic substrates occurs proceeds through a concerted proton–electron transfer from the aquo ligand. Atomistic resolution of surface reactivity presents a novel route of hydrogenation reactivity from metal oxide surfaces through H-atom transfer from surface-bound water molecules.


 Please wait while we load your content...
Please wait while we load your content...