From peptides to proteins: coiled-coil tetramers to single-chain 4-helix bundles†
Abstract
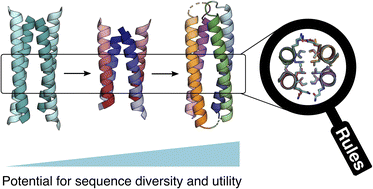
The design of completely synthetic proteins from first principles—de novo protein design—is challenging. This is because, despite recent advances in computational protein–structure prediction and design, we do not understand fully the sequence-to-structure relationships for protein folding, assembly, and stabilization. Antiparallel 4-helix bundles are amongst the most studied scaffolds for de novo protein design. We set out to re-examine this target, and to determine clear sequence-to-structure relationships, or design rules, for the structure. Our aim was to determine a common and robust sequence background for designing multiple de novo 4-helix bundles. In turn, this could be used in chemical and synthetic biology to direct protein–protein interactions and as scaffolds for functional protein design. Our approach starts by analyzing known antiparallel 4-helix coiled-coil structures to deduce design rules. In terms of the heptad repeat, abcdefg—i.e., the sequence signature of many helical bundles—the key features that we identify are: a = Leu, d = Ile, e = Ala, g = Gln, and the use of complementary charged residues at b and c. Next, we implement these rules in the rational design of synthetic peptides to form antiparallel homo- and heterotetramers. Finally, we use the sequence of the homotetramer to derive in one step a single-chain 4-helix-bundle protein for recombinant production in E. coli. All of the assembled designs are confirmed in aqueous solution using biophysical methods, and ultimately by determining high-resolution X-ray crystal structures. Our route from peptides to proteins provides an understanding of the role of each residue in each design.
- This article is part of the themed collections: Computational protein design and structure prediction: Celebrating the 2024 Nobel Prize in Chemistry and 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...